Expected Revenue Growth:
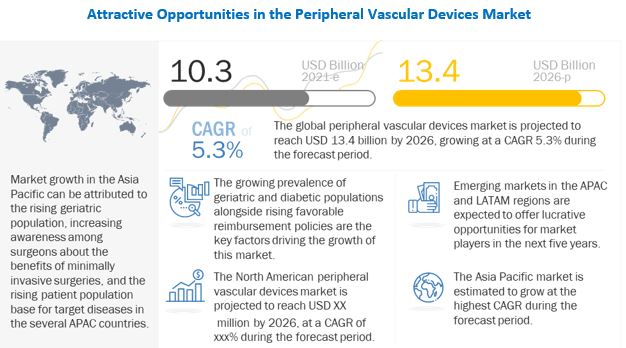
[347 Pages Report] The global peripheral vascular devices market size is projected to reach USD 13.4 billion by 2026 from USD 10.3 billion in 2021, at a CAGR of 5.3% from 2021 to 2026.
Download PDF Brochure @ https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=790
What This Report Will Provide?
The study involved four major activities in estimating the current size of the peripheral vascular devices market. Exhaustive secondary research was done to collect information on the market and its different subsegments.
The next step was to validate these findings, assumptions, and sizing with industry experts across the value chain through primary research. Both top-down and bottom-up approaches were employed to estimate the complete market size.
Major Growth Boosters:
The growth of this market is driven by rapid growth in the geriatric population and the subsequent increase in the prevalence of peripheral vascular diseases, favorable reimbursement scenario for peripheral vascular procedures, Increased number of product approvals, increased prevalence of diabetes, and rising rate of tobacco consumption.
Regional Growth Analysis:
The peripheral vascular devices market is segmented into four major regional segments, namely, North America, Europe, Asia Pacific, and the Rest of the World. In 2021, North America accounted for the largest share of the peripheral vascular devices market. The large share of North America can be attributed to the growth in the geriatric population and the high prevalence of peripheral vascular diseases. Furthermore, the presence of key market players in this region ensures high access to medical devices
Key Market Players
The major players in the peripheral vascular devices market Medtronic (Ireland), Boston Scientific Corporation (US), Abbott (US), Becton, Dickinson and Company (US), Terumo Corporation (Japan), Cardinal Health (US), B. Braun Melsungen AG (US), Merit Medical Systems (US), Penumbra, Inc.(US), Koninklijke Philips N.V.( Netherlands), iVascular S.L.U (Spain), Biosensors International Group Ltd. (Singapore), BIOTRONIK (Germany), AMG International GmbH (US), ENDOCOR GmbH (Germany).
Request Sample Report: https://www.marketsandmarkets.com/requestsampleNew.asp?id=790
Recent Developments:
- Medtronic received the US FDA approval for its IN. PACT AV drug-coated balloon (DCB) to treat arteriovenous fistula lesions
- Boston Scientific Corporation received the FDA Approval for its VICI VENOUS STENT System for treating patients with deep venous blockages.
Frequently Asked Questions (FAQ):
- What is the projected market value of the global peripheral vascular devices market?
- What is the estimated growth rate (CAGR) of the global peripheral vascular devices market for the next five years?
- Who are the major players offering peripheral vascular devices in the market?
To speak to our analyst for a discussion on the above findings @ https://www.marketsandmarkets.com/speaktoanalystNew.asp?id=790








