Market Scope
On the basis of indication, the Asia-Pacific IVD regulatory affairs outsourcing market is segmented into oncology, neurology, cardiology, clinical chemistry and immunoassays, precision medicine, infectious diseases, diabetes, genetic testing, HIV/AIDS, haematology, drug testing/pharmacogenomics, blood transfusion, point of care, and others. In 2022, the oncology segment is expected to dominate as high quality technology and service portfolio related to the IVD regulatory application draws more involvement of the peer.
Get the sample copy of Report here: https://www.databridgemarketresearch.com/request-a-sample?dbmr=asia-pacific-ivd-regulatory-affairs-outsourcing-market
Asia-Pacific IVD Regulatory Affairs Outsourcing Market Regional Analysis/Insights
In vitro diagnostic products are reagents, devices, and systems used to diagnose disease or other conditions, including determining one's state of health to cure, mitigate, treat, or prevent disease. These products are intended for use in the collecting, preparation, and examination of human body specimens. Regulatory affairs play a crucial part in the in vitro diagnostic device (IVD) and medical device industry. The regulatory affairs outsourcing services entails medical writing and publication of regulatory documentation by professionals who contribute to the production of high-quality documents for clinical research projects. The demand for regulatory services outsourcing is substantially increasing in clinical studies conducted in emerging economies, providing a healthy platform for this industry's growth.
This IVD regulatory affairs outsourcing market report provides details of market share, new developments, and product pipeline analysis, impact of domestic and localised market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, product approvals, strategic decisions, product launches, geographical expansions, and technological innovations in the market. To understand the analysis and the market scenario contact us for an Analyst Brief, our team will help you create a revenue impact solution to achieve your desired goal.
Asia-Pacific IVD Regulatory Affairs Outsourcing Market Country Level Analysis
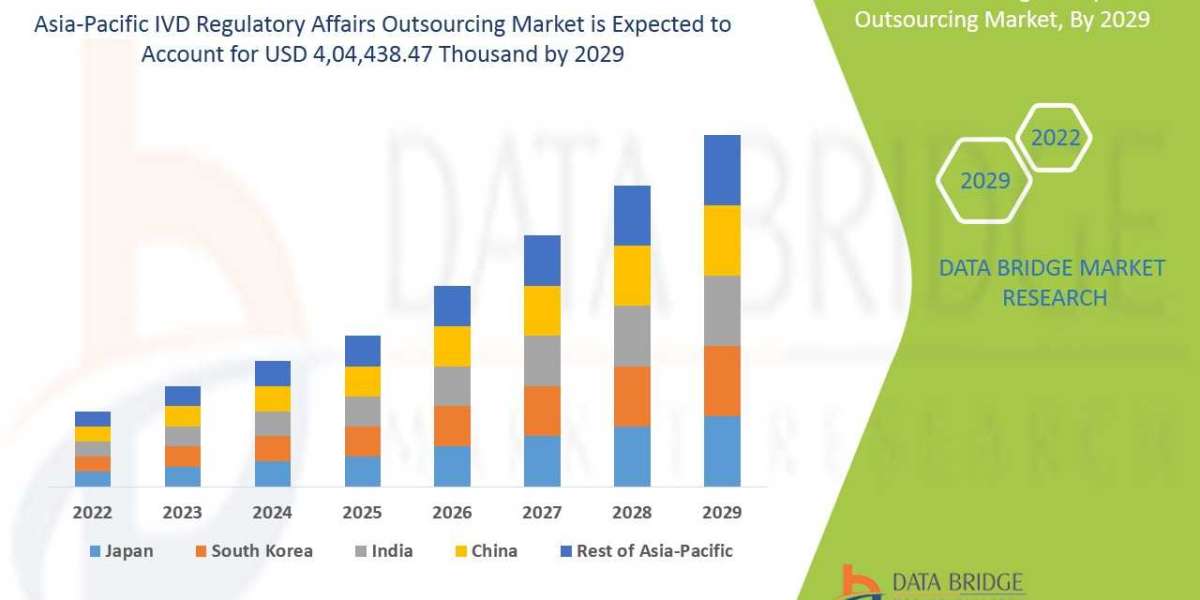
Asia-Pacific IVD regulatory affairs outsourcing market is analysed and market size information is provided by the country, services, indication, deployment mode, organization size, stage, class and end user.
The countries covered in Asia-Pacific IVD regulatory affairs outsourcing market report are China, South Korea, Japan, India, Australia, Singapore, Malaysia, Indonesia, Thailand, Philippines and Rest of Asia-Pacific.
China is expected to dominate the market in Asia-Pacific region due to increasing developments in the healthcare sector and availability of the largest clinical laboratory in Asia-Pacific.
Asia-Pacific IVD Regulatory Affairs Outsourcing Market Share Analysis
Asia-Pacific IVD regulatory affairs outsourcing market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, company strengths and weaknesses, product launch, product trials pipelines, product approvals, patents, product width and breadth, application dominance, technology lifeline curve. The above data points provided are only related to the companies’ focus related to Asia-Pacific IVD regulatory affairs outsourcing market.
Key Players Asia-Pacific IVD Regulatory Affairs Outsourcing Market
- Freyr Solutions
- PPD Inc.(A Subsidiary of Thremofisher Scientific Inc.)
- EMERGO
- ICON
- Parexel International Corporation
- CRITERIUM INC.
- Groupe ProductLife S.A.
- Labcorp Drug Development
- WuXi AppTec
- Genpact, Medpace
- Dor Pharmaceutical Services
- Qserve
Get Full Access of Report @ https://www.databridgemarketresearch.com/reports/asia-pacific-ivd-regulatory-affairs-outsourcing-market
MAJOR TOC OF THE REPORT
- Chapter One: Introduction
- Chapter Two: Market Segmentation
- Chapter Three: Market Overview
Get TOC Details: https://www.databridgemarketresearch.com/toc/?dbmr=asia-pacific-ivd-regulatory-affairs-outsourcing-market
Browse Related Repord @
Global IVD Regulatory Affairs Outsourcing Market
Middle East and Africa Asia-Pacific IVD Regulatory Affairs Outsourcing Market
North America Asia-Pacific IVD Regulatory Affairs Outsourcing Market
Europe Asia-Pacific IVD Regulatory Affairs Outsourcing Market
Global in Vitro Diagnostic (IVD) Regulatory Affairs Outsourcing Market
BROWSE RELATED BLOGS @
BROWSE RELATED INFOGRAPHICS
About Us:
Data Bridge Market Research set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market
Contact:
Data Bridge Market Research
Tel: +1-888-387-2818