The pharmaceutical industry has continued to forge strategic partnerships with vendors of Artificial Intelligence Market throughout 2021 and the first quarter of 2022. Merck's most recent wager is to use digitally simulated "predicted outcomes" rather than actual patients to generate clinical evidence that can be used to support regulatory decisions. Unlearn and the pharmaceutical company have agreed to work together for many years. By enrolling "Digital Twins," virtual replicas of existing patients, AI, a startup, promises to cut clinical research time and costs while maintaining trial power and confidence.
NASA created the first Digital Twin to replicate Apollo 13, providing Houston engineers with information about the rocket's predicted health and trajectory. A Digital Twin could be described as "an experimental model recapitulating the complexity of a system that, in response to an event, behaves in a manner statistically indistinguishable from its real counterpart" using terms from biomedicine.
Unlearn. The Digital Twin technology of Artificial Intelligence Market is based on two exclusive pipelines. The first, PROCOVA, is a machine learning technique that uses historical datasets to find prognostic markers and scores and create a generative model that can predict how a disease will develop clinically. Twintelligent RCTs, the second, is an AI-powered algorithm that compares a patient's baseline clinical records to the generative model to predict a comprehensive, longitudinal clinical record that explains what would have occurred if that patient had been given a placebo. The number of patients required for the study is cut by 30% using this strategy. A final quality control algorithm that, absent bias, should not be able to distinguish the real dataset from the simulated dataset still requires some real control patients.
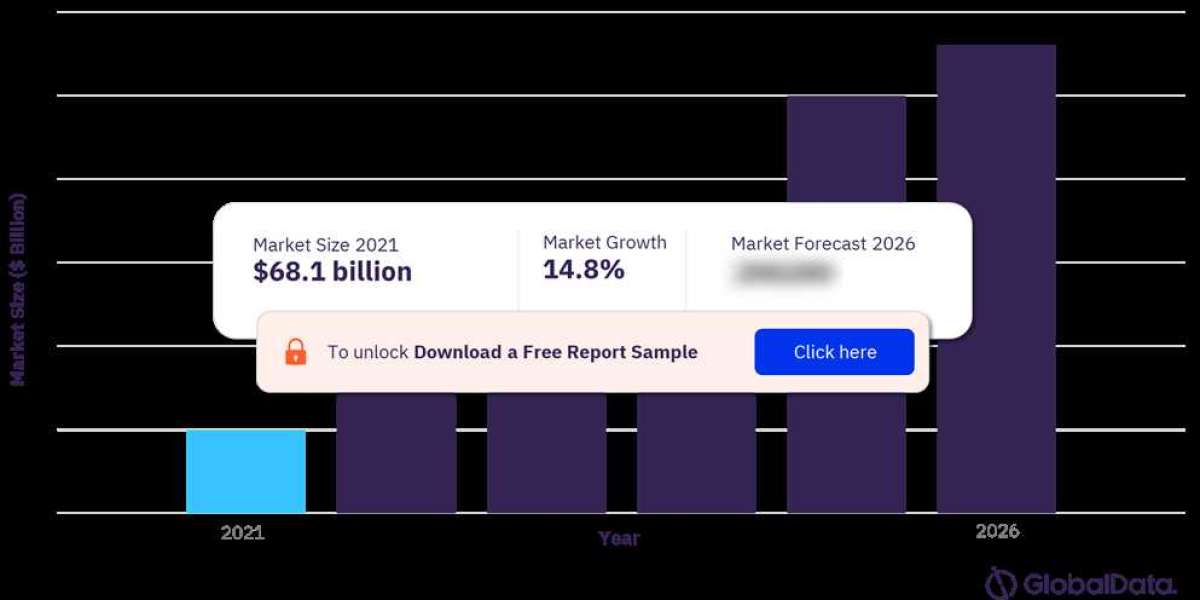
For more insights on the Artificial Intelligence Market, Download a Free Sample
By avoiding the use of external comparators, this method reduces bias in comparison to the existing synthetic control arms (SCA). Because it is a univariate analysis in which the only difference between the patients and their twins is the treatment they receive, this strategy is theoretically superior to even the gold-standard randomised controlled trials. Additionally, PROCOVA has a unique advantage: The generative model does not attempt to justify a patient's survival solely on the basis of their clinical profile. Instead, it makes room for both known and unknown confounding variables, which will always cause a diagnostic forecast to be off from reality. Therefore, the AI elegantly controls and corrects this bias without having to fully characterize human complexity by modeling uncertainty. The US Food and Drug Administration (FDA), which was actively involved by the developers, has commended the pipeline's ability to control bias.
The European Medicines Agency (EMA) also issued a preliminary approval for PROCOVA on March 22, 2022, allowing this technology to be used in European Union Phase II and III trials.
Unlearn on April 19th. To advance the use of Twintelligent RCTs in clinical trials, AI closed a $50 million Series B funding. The AI vendor's training ground, the company has built databases that cover disorders of the central nervous system (CNS) and several therapeutic applications that reflect Merck's upcoming products. The CEO of Unlearn mentioned a second phase of the collaboration, which could suggest that the goal is to export Digital Twins to all of the therapeutic applications covered. So, what do these things mean for oncology?
When a control arm is difficult or unethical, like in pediatric settings, the FDA currently considers SCA to be the best scientific evidence. AI would likely take the place of external control groups if the FDA's guidelines for using AI in clinical trials were made official.
However, it appears more complicated to use Digital Twins in more late-phase trials. Since investigational new drugs and standard treatments are compared in oncology, each twin would serve as both a prognostic and predictive forecast. For every treatment plan, classification, and position, among other things, distinct generative models would be required. Cancer would necessitate at least three -omics datasets due to its complexity (likely copy number variation, methylation status, and RNA-seq), and the disease's dynamic evolution would quickly invalidate the model and necessitate a new one. However, in addition to its primary intended application, this machine learning technique may have two specific characteristics that have the potential to have a significant impact on oncology.
First, the model continues to learn about the disease for each new patient; Second, PROCOVA is ideal for predicting the likelihood that any one of several possible causes contributed to an event because of the way the Bayesian network makes predictions. In a wide range of treatment settings, 1,700 clinical trials are currently attempting to identify patients who are likely to respond to the PD-1 inhibitor Keytruda. In addition to enabling smaller, more effective trials that maintain power while also resulting in quicker and less expensive therapies for patients, the application of AI and machine learning can offer potent tools for answering these questions.