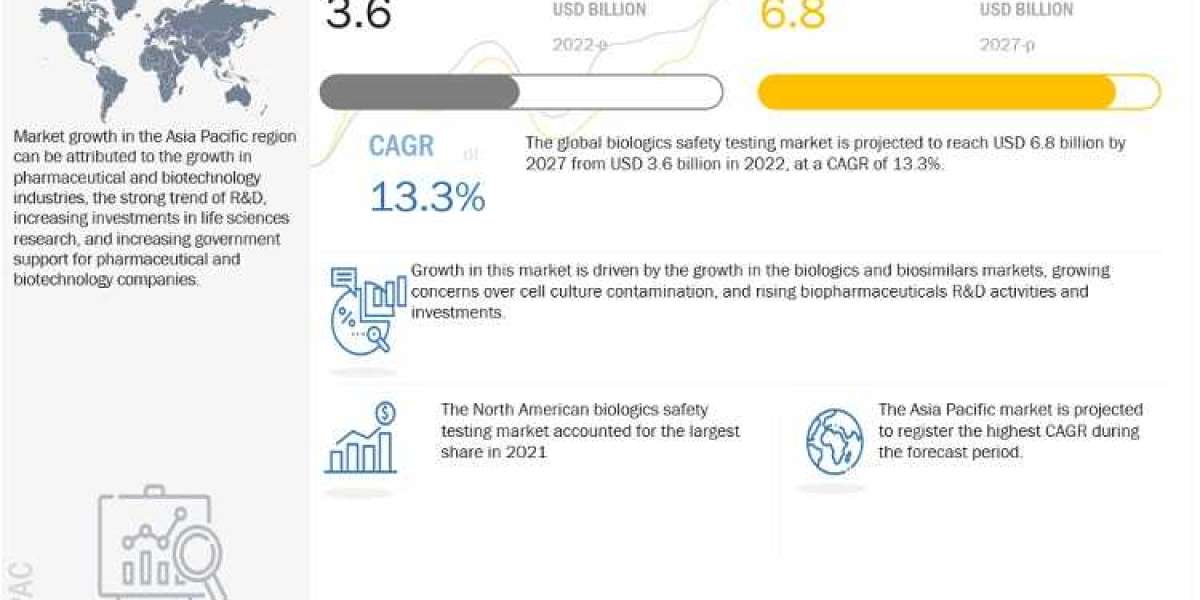
Biologics Safety Testing Market is projected to reach USD 6.8 billion by 2027 from USD 3.6 billion in 2022, at a CAGR of 13.3% during the forecast period according to a new report by MarketsandMarkets™. The growth of the global biologics safety testing market is driven by factors such as companies investing heavily in the development of biologics and biosimilars, strict regulatory concerns over growing cell culture contamination, and increased demand for various drugs, cell therapies, diagnostics, and active biological products.
Download PDF Brochure: https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=34624144
Browse in-depth TOC on "Biologics Safety Testing Market"
185 - Tables
46 - Figures
249 – Pages
Key Market Players
Prominent players in the biologics safety testing market are Charles River Laboratories, Inc. (US), Lonza (Switzerland), Thermo Fisher Scientific, Inc. (US), Merck KGaA (Germany), Labcorp (US), F. Hoffmann-La Roche Ltd. (Switzerland), Agilent Technologies, Inc. (US), Sartorius AG (Germany), and Lonza (Switzerland). The key players in this market are focusing on strategic expansions, partnerships, and product service launches and approvals to expand their presence in the market.
CHARLES RIVER LABRATORIES (US)
Charles River is one of the key players in the biologics safety testing market. It is a service and early-stage contract research organization (CRO). The company develops a diverse portfolio of discovery safety assessment services by providing a suite of products and services to support its clients’ manufacturing activities. The company operates through three reporting segments, namely, Research Models and Services (RMS), Discovery and Safety Assessment (DSA), and Manufacturing Support (Manufacturing). The company offers biologics safety testing solutions through its Manufacturing Support segment. To further strengthen its market position, Charles River Laboratories focuses on expanding its service and product offerings in the biologics safety testing segment. The company offers customized assays to address specific concerns and needs, ensuring an appropriate testing plan is designed for biological products. Fast Track Testing is also available to quickly deliver urgently needed results. To maintain its leading position and enhance its market share, the company mainly focuses on exceeding customer expectations by maintaining the quality of its services and quickly adapting to new conditions with efficiency. The acquisitions of JADE Biomedical (China) and Cognate BioServices (US) have further strengthened the company’s portfolio and geographic presence in the market.
LONZA (SWITZERLAND)
Lonza is a key player in the biologics safety testing market. The company has a large number of manufacturing and RD facilities worldwide. The company's wide geographic presence and high production capacity deliver quality products worldwide. The company focuses on organic growth strategies to maintain its position in the biologics safety testing market. In line with this, in April 2021, Lonza launched the PyroTec PRO Automated Robotic Solution, designed for use with the sustainable PyroGene Recombinant Factor C (rFC) Assay for endotoxin testing. Such developments have helped the company enhance its visibility in the biologics safety testing market.
THERMO FISCHER SCIENTIFIC (US)
Thermo Fisher Scientific offers analytical instruments, equipment, reagents, and consumables for research, discovery, analysis, diagnostics, and manufacturing. The company operates through four principal product segments—Life Sciences Solutions, Analytical Instruments, Specialty Diagnostics, and Laboratory Products and Services. The company offers a wide array of biologics safety testing products through its Life Sciences Solutions segment. The company offers a wide range of biological safety testing kits for endotoxin testing, residual host-cell proteins DNA detection tests, mycoplasma tests, and virus safety testing for biologics molecules.
Request Sample Pages: https://www.marketsandmarkets.com/requestsampleNew.asp?id=34624144
The Asia Pacific region is the fastest growing region of the Biologics safety testing market
The Asia Pacific market is projected to witness the highest growth rate during the forecast period. Rapid growth in outsourcing preclinical, clinical, and laboratory testing services to APAC countries is expected to drive market growth during the forecast period. Several companies are focusing on increasing their market shares and customer base in the region to capitalize on these opportunities in the Asia Pacific market.
Recent Developments:
- In November 2022, Merck KGaA (Germany) invested in expanding its biosafety testing capacity at Rockville, Maryland, US. This site will provide biosafety testing and analytical development services in the US.
- In July 2022, Eurofins Scientific (Luxembourg) acquired Wessling Hungary, a food, environmental, and BioPharma product testing laboratory in Hungary. This acquisition helped expand Eurofins' biopharma testing business in Europe.
- In June 2022, Lonza (Switzerland) launched the PyroCell Monocyte Activation Test to detect non-endotoxin pyrogens and reduce interferences from complex drug products, such as biologics-based pharmaceuticals.
- In March 2021 Charles River Laboratories, Inc. (US) launched a new detection tool, EndoScan-V, a validated endotoxin detection and measurement software used to generate and report quantitative test data. The software performed the requisite measurements and calculations and created test reports with the convenience of digital signature report approval.
- In January 2021, Charles River Laboratories, Inc. (US) partnered with JADE Biomedical to expand its biological testing solutions capabilities geographically and cater to the increasing demand for biologics therapeutics, especially cell and gene therapies. This strategic relationship enabled JADE to expand its current global Good Manufacturing Practice (GMP) product testing operations in Shanghai into a second facility and further build upon its current offering of comprehensive biologics quality management and testing services.
Get 10% Free Customization on this Report: https://www.marketsandmarkets.com/requestCustomizationNew.asp?id=34624144
About MarketsandMarkets™:
MarketsandMarkets™ is a blue ocean alternative in growth consulting and program management, leveraging a man-machine offering to drive supernormal growth for progressive organizations in the B2B space. We have the widest lens on emerging technologies, making us proficient in co-creating supernormal growth for clients.
The B2B economy is witnessing the emergence of $25 trillion of new revenue streams that are substituting existing revenue streams in this decade alone. We work with clients on growth programs, helping them monetize this $25 trillion opportunity through our service lines - TAM Expansion, Go-to-Market (GTM) Strategy to Execution, Market Share Gain, Account Enablement, and Thought Leadership Marketing.
Built on the 'GIVE Growth' principle, we work with several Forbes Global 2000 B2B companies - helping them stay relevant in a disruptive ecosystem. Our insights and strategies are molded by our industry experts, cutting-edge AI-powered Market Intelligence Cloud, and years of research. The KnowledgeStore™ (our Market Intelligence Cloud) integrates our research, facilitates an analysis of interconnections through a set of applications, helping clients look at the entire ecosystem and understand the revenue shifts happening in their industry.